Step-by-step explanation:
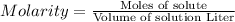
A. 2.00 mL of 0.00250 M
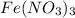
Moles of ferric nitrate = n
Volume of ferric nitrate = 2.00 ml = 0.002 L ( 1 mL=0.001 L)
Molarity of ferric nitrate = 0.00250 M
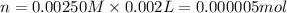
B. 5.00 mL of 0.00250 M
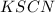
Moles of KSCN = n'
Volume of KSCN = 5.00 ml = 0.005 L ( 1 mL=0.001 L)
Molarity of KSCN = 0.00250 M
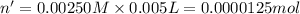
C. 3.00 mL of 0.050 M
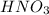
Moles of nitric acid = n''
Volume of nitric acid = 3.00 ml = 0.003 L ( 1 mL=0.001 L)
Molarity of nitric acid = 0.050 M
After mixing A, B and C together and their respective initial concentration before reaction.
After mixing A, B and C together the volume of the solution becomes = V
V = 0.002 L=0.005 L+0.003 L= 0.010 L
Concentration of ferric nitrate :
![[Fe(NO_3)_3]=(0.000005 mol)/(0.010 L)=0.0005 M](https://img.qammunity.org/2021/formulas/chemistry/college/4dyspbv8o7c3murzxlybymrcs8bvihpfh6.png)
Concentration of ferric ions :
![[Fe^(3+)]=1* [Fe(NO_3)_3]=0.0005 M](https://img.qammunity.org/2021/formulas/chemistry/college/9k2r0wr5ar37zr2h3h4u87txx7hv8o2aad.png)
Concentration of nitrate ions from ferric nitrate:
![[NO_3^(-)]=3* [Fe(NO_3)_3]=0.0015 M](https://img.qammunity.org/2021/formulas/chemistry/college/t25rgro32zdp8dx2mflpcpew349ef7hz9i.png)
Concentration of KSCN :
![[KSCN]=(0.0000125 mol)/(0.010 L)=0.00125 M](https://img.qammunity.org/2021/formulas/chemistry/college/om9apztlvjkkgkw3izzdclihybmitaami0.png)
Concentration of
 ions:
ions:
![[SCN^-]=1* [KSCN]=0.00125 M](https://img.qammunity.org/2021/formulas/chemistry/college/a4hfj6iskz8dgtqwdtu7jup66yzw6o701r.png)
Concentration of potassium ions:
![[K^+]=1* [KSCN]=0.00125 M](https://img.qammunity.org/2021/formulas/chemistry/college/j75ol4932rp8vja1w387vje7xb5r8jn1pt.png)
Concentration of nitric acid :
![[HNO_3]=(0.00015 mol)/(0.010 L)=0.015 M](https://img.qammunity.org/2021/formulas/chemistry/college/llu0qj7kx25p8k7d64dl0y6xj94y7buifx.png)
Concentration of hydrogen ion :
![[H^+]=1* [HNO_3]=0.015 M](https://img.qammunity.org/2021/formulas/chemistry/college/p8a13uq7xcdlkbzlkox9vjzmvonvc3x5v0.png)
Concentration of nitrate ions from nitric acid :
![[NO_3^(-)]=1* [HNO_3]=0.0015 M](https://img.qammunity.org/2021/formulas/chemistry/college/la7o3cga8vgr85yhb8a3defn9m5v050z7f.png)
Concentration of nitrate ion in mixture = 0.0015 M + 0.0015 M = 0.0030 M
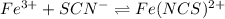
given concentration of
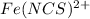 at equilbrium =
at equilbrium =
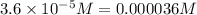
initially :
0.0005 M 0.00125 M 0
At equilibrium
(0.0005-0.000036) M (0.00125-0.000036) M 0.000036 M
0.000464 M 0.001214 M 0.000036 M
The expression of an equilibrium constant will be given as;
![K_c=([Fe(NCS)^(2+)])/([Fe^(3+)][SCN^(-)])](https://img.qammunity.org/2021/formulas/chemistry/college/j8s2j3cf8ah0bp3a33szn2bnotp9k76abf.png)
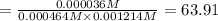
The value for the equilibrium constant is 63.91.