Answer:
(A) three times as large as the initial value.
Step-by-step explanation:
Boyle law states that the pressure is inversely proportional to volume if we kept the temperature constant.
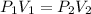 (1)
(1)
here
 and
and
 are the initial pressure and volume.
are the initial pressure and volume.
 and
and
 are the final pressure and volume respectively.
are the final pressure and volume respectively.
now
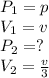
by putting these values in equation 1.
hence it is proved that " if the volume is decreased to one third of its original value then the pressure will be three times larger then its initial value".