Answer:
pH of HCl solution is 1.44
Step-by-step explanation:
NaOH is a monoprotic base and HCl is a monobasic acid.
Neutralization reaction:

According to balanced reaction, 1 mol of NaOH neutralizes 1 mol of HCl.
Number of moles of NaOH in 13 mL of 0.21 M NaOH
=
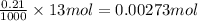
let's assume concentration of HCl is C (M)
Then, number of moles of HCl in 75 mL of C (M) HCl solution
=
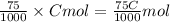
So, we can write,
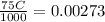
or,
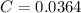
1 mol of HCl contains 1 mol of

So, concentration of
 in 0.0364 M HCl,
in 0.0364 M HCl,
![[H^(+)]=0.0364M](https://img.qammunity.org/2021/formulas/chemistry/high-school/z4ehidbbji5wxziyv4b64b8ud0938iq1js.png)
Hence,
![pH=-log[H^(+)]=-log(0.0364)=1.44](https://img.qammunity.org/2021/formulas/chemistry/high-school/6d2azsvqzuepgj7gt3ws0mkd16ly5nzls4.png)