The question is incomplete, here is the complete question:
A solution is made by mixing of 51 g of heptane and 127 g of acetyl bromide. Calculate the mole fraction of heptane in this solution. Round your answer to 3 significant digits.
Answer: The mole fraction of heptane in the solution is 0.330
Step-by-step explanation:
To calculate the number of moles, we use the equation:
 .....(1)
.....(1)
Given mass of heptane = 51 g
Molar mass of heptane = 100.2 g/mol
Putting values in equation 1, we get:
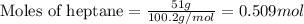
Given mass of acetyl bromide = 127 g
Molar mass of acetyl bromide = 123 g/mol
Putting values in equation 1, we get:
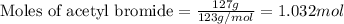
Mole fraction of a substance is given by:
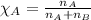
Moles of heptane = 0.509 moles
Total moles = [0.509 + 1.032] = 1.541 moles
Putting values in above equation, we get:
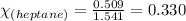
Hence, the mole fraction of heptane in the solution is 0.330