Answer: Option (A) is the correct answer.
Step-by-step explanation:
Exothermic reaction is defined as the reaction in which release of heat takes place. Whereas endothermic reaction is defined as the reaction in which heat is absorbed by the reactant molecules.
- When ice melts at room temperature it is an endothermic reaction as it occurs due to absorption of heat.
- Boiling of water at
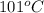 is also an endothermic process. This is because heat is absorbed by water molecules due to which their state changes from liquid to vapor form.
is also an endothermic process. This is because heat is absorbed by water molecules due to which their state changes from liquid to vapor form.
- When
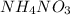 is dissolved in water then heat is absorbed as there occurs a decrease in temperature of water. Hence, it is also an endothermic reaction.
is dissolved in water then heat is absorbed as there occurs a decrease in temperature of water. Hence, it is also an endothermic reaction.
Thus, we can conclude that for the given statements its is true that ALL three of the given processes are endothermic.