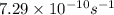The question is incomplete, here is the complete question:
1. The radioactive source you will be working with in this lab is Cs-137. Look up the half-life of this material and report the value in units of seconds.
2. The relationship between decay constant (l) and half-life is:
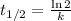
For Cs-137, what is the value of 'k' in
Answer:
For 1: The half life for Cs-137 isotope is

For 2: The rate constant of Cs-137 isotope is
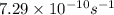
Step-by-step explanation:
Half life is defined as the time taken for half of the reaction to complete. This is also defined as the time in which the concentration of a reactant is reduced to half of its original value.
The half life for Cs-137 isotope is

The relationship between decay constant (l) and half-life is: given by the equation:
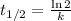
where,
 = half life of Cs-137 isotope =
= half life of Cs-137 isotope =

k = rate constant
Putting values in above equation, we get:
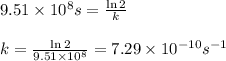
Hence, the rate constant of Cs-137 isotope is