Answer: The average rate of the reaction during this time interval is, 0.005 M/s
Step-by-step explanation:
The given chemical reaction is:
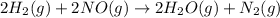
The expression will be:
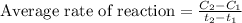
where,
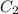 = final concentration of
= final concentration of
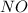 = 0.025 M
= 0.025 M
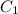 = initial concentration of
= initial concentration of
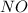 = 0.100 M
= 0.100 M
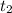 = final time = 15 minutes
= final time = 15 minutes
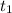 = initial time = 0 minutes
= initial time = 0 minutes
Putting values in above equation, we get:
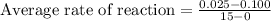
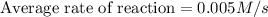
Hence, the average rate of the reaction during this time interval is, 0.005 M/s