The question is incomplete, here is the complete question:
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water. If 12.5 g of water is produced from the reaction of 72.6 g of sulfuric acid and 77.0 g of sodium hydroxide, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it.
Answer: The percent yield of water in the reaction is 46.85 %.
Step-by-step explanation:
To calculate the number of moles, we use the equation:
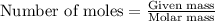 .....(1)
.....(1)
Given mass of NaOH = 77.0 g
Molar mass of NaOH = 40 g/mol
Putting values in equation 1, we get:
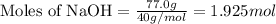
Given mass of sulfuric acid = 72.6 g
Molar mass of sulfuric acid = 98 g/mol
Putting values in equation 1, we get:
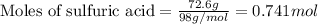
The chemical equation for the reaction of NaOH and sulfuric acid follows:
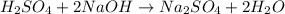
By Stoichiometry of the reaction:
1 mole of sulfuric acid reacts with 2 moles of NaOH
So, 0.741 moles of sulfuric acid will react with =
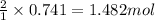 of NaOH
of NaOH
As, given amount of NaOH is more than the required amount. So, it is considered as an excess reagent.
Thus, sulfuric acid is considered as a limiting reagent because it limits the formation of product.
By Stoichiometry of the reaction:
1 mole of sulfuric acid produces 2 moles of water
So, 0.741 moles of sulfuric acid will produce =
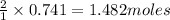 of water
of water
Now, calculating the mass of water from equation 1, we get:
Molar mass of water = 18 g/mol
Moles of water = 1.482 moles
Putting values in equation 1, we get:
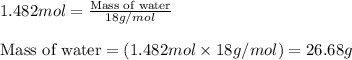
To calculate the percentage yield of water, we use the equation:
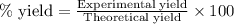
Experimental yield of water = 12.5 g
Theoretical yield of water = 26.68 g
Putting values in above equation, we get:
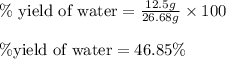
Hence, the percent yield of water in the reaction is 46.85 %.