Answer:
The average rate of energy transfer to the cooker is 1.80 kW.
Step-by-step explanation:
Given that,
Pressure of boiled water = 300 kPa
Mass of water = 3 kg
Time = 30 min
Dryness friction of water = 0.5
Suppose, what is the average rate of energy transfer to the cooker?
We know that,
The specific enthalpy of evaporate at 300 kPa pressure
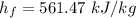
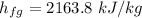
We need to calculate the enthalpy of water at initial state
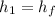
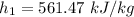
We need to calculate the enthalpy of water at final state
Using formula of enthalpy
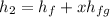
Put the value into the formula
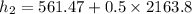
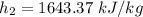
We need to calculate the rate of energy transfer to the cooker
Using formula of rate of energy
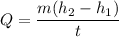
Put the value into the formula
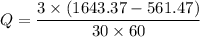
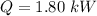
Hence, The average rate of energy transfer to the cooker is 1.80 kW.