Step-by-step explanation:
When a substance or chemical specie accepts a proton then an acid is formed. Whereas is a substance or chemical specie tends to lose a proton then a base is formed.
Chemical formula of amino group is
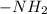 and it is able to lose a proton to form R-NH-R'. When it tends to gain a proton the it form R-NH_{3} compound.
and it is able to lose a proton to form R-NH-R'. When it tends to gain a proton the it form R-NH_{3} compound.
Chemical formula of carboxylic group is -COOH. When it loses a proton it forms
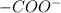 ion and whenit gains an electron then it forms
ion and whenit gains an electron then it forms
 .
.
Therefore, we can conclude that the amino and carboxyl functional groups tend to form bases and acids by attracting or dropping a proton.