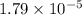Answer : The value of
 of the generic salt AB is,
of the generic salt AB is,
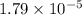
Explanation : Given,
Concentration of generic cation,
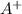 = 0.00423 M
= 0.00423 M
Concentration of generic anion,
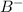 = 0.00423 M
= 0.00423 M
The equilibrium chemical reaction will be:
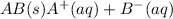
The solubility constant expression for this reaction is:
![K_(sp)=[A^+][B^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/o0wwhke5eq8zwexe14tvpv6r7e1y8hw9lp.png)
Now put all the given values in this expression, we get:
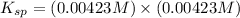
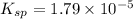
Thus, the value of
 of the generic salt AB is,
of the generic salt AB is,