The question is incomplete, here is the complete question:
What volume in mL of your HCl solution did you use for each of the three acceptable trial samples for the standardization?
Trials Molarity of HCl Volume of NaOH
1 0.1 M 24.80 mL
2 0.2 M 19.20 mL
3 0.01 M 18.00 mL
The average molarity of NaOH is 0.0755 M
Answer:
For Trial 1: The volume of HCl required is 18.72 mL
For Trial 2: The volume of HCl required is 7.25 mL
For Trial 3: The volume of HCl required is 135.9 mL
Step-by-step explanation:
To calculate the volume of acid, we use the equation given by neutralization reaction:
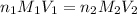
where,
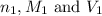 are the n-factor, molarity and volume of acid which is HCl
are the n-factor, molarity and volume of acid which is HCl
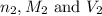 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.
We are given:
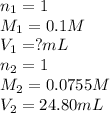
Putting values in above equation, we get:
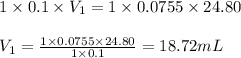
Hence, the volume of HCl required is 18.72 mL
We are given:
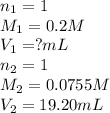
Putting values in above equation, we get:
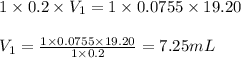
Hence, the volume of HCl required is 7.25 mL
We are given:
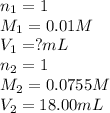
Putting values in above equation, we get:
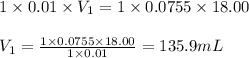
Hence, the volume of HCl required is 135.9 mL