Answer : The minimum amount of 6.9 M
 needed is, 2.0 L
needed is, 2.0 L
Explanation :
The given chemical reaction is:
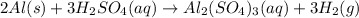
First we have to calculate the moles of

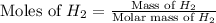
Molar mass of
 = 2 g/mol
= 2 g/mol
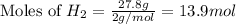
Now we have to calculate the moles of

From the balanced chemical reaction we conclude that,
As, 3 moles of
 produced from 3 moles of
produced from 3 moles of

So, 13.9 moles of
 produced from 13.9 moles of
produced from 13.9 moles of

Now we have to calculate the mass of

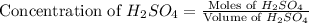
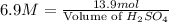
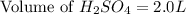
Thus, the minimum amount of 6.9 M
 needed is, 2.0 L
needed is, 2.0 L