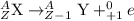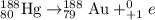Answer: The daughter nucleus produced when 188 Hg undergoes positron emission is gold.
Step-by-step explanation:
Positron emission: It is a type of decay process, in which a proton gets converted to neutron and an electron neutrino. This is also known as -decay. In this the mass number remains same.
General representation of an element is given as:
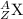
where,
Z represents Atomic number
A represents Mass number
X represents the symbol of an element
The chemical equation for positron emission is represented as: