Answer:
The concentration of hydrogen ions is 0.07 M
Step-by-step explanation:
HCl is a strong acid. When it dissolves in water, it dissociates completely in H⁺ and Cl⁻ ions.
From the definition of pH:
pH = - log [H⁺]
We introduce the pH of the solution (1.14) and calculate the concentration of hydrogen ions ([H⁺]) in M :
[H⁺] =
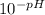 =
=
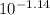 = 0.0724 M= 0.07 M
= 0.0724 M= 0.07 M