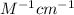Answer:
the value of molar absorptivity is 229000
Step-by-step explanation:
given data
phenol phthalein solution = 0.050 g
total volume = 100.0 ml
dilute = 100.0 ml
diluted sample = 0.18
solution
we get here concentration that is express as
concentration = ( mass of solute × 1000 ) ÷ ( molar mass of solute × volume of solution ) .............1
put here value
concentration =
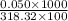
concentration = 0.00157 M
and here dillution equation is express as
c1 × v1 = c2 × v2 .................2
here c1 and c2 is initial and final concentration
and v1 and v2 is initial and final volume
put here value
0.001571 × 0.050 = c2 × 100
c2 = 7.855 ×
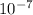 M
M
and
now we get molar by absorbance equation that is
A = E × C × l ................3
here A is absorbance and E is molar and c is absorptivity and l is path length
put here value
0.18 = E × 7.855 ×
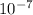 × 1
× 1
E = 229000