Answer:
6.75 × 10⁻⁸is the value of the equilibrium constant at this temperature.
Step-by-step explanation:
2H₂O(g) ⇄ 2H₂(g) + O₂(g)
Partial pressure of H₂O = 0.0500 atm
Partial pressure of H₂ = 0.00150 atm
Partial pressure of O₂ = 0.00150 atm
The expression of Kp for the given chemical equation is:
![K_p = ([H_2]^2[O_2])/(H_2O)](https://img.qammunity.org/2021/formulas/chemistry/college/ht9fvtbozt4jqak5mndiq3kj6mlhs3875c.png)
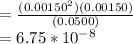
6.75 × 10⁻⁸is the value of the equilibrium constant at this temperature