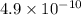Answer : The solution that has highest hydronium ion concentration is, (B) HCN,
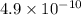
Explanation :
As we know that:
![[H^+]=√((K_aC))](https://img.qammunity.org/2021/formulas/chemistry/college/n5605y5mq0t17zut3iqfryn8mygpf4e0eg.png)
And,
pH : It is defined as the negative logarithm of hydrogen ion or hydronium ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2021/formulas/chemistry/high-school/rjo2yhb5oj9ry1fr4db1ujrazm6fh3vhke.png)
From this we conclude that, as the value of
 (dissociation constant) increases then the value of hydrogen ion concentration also increases and the value of pH decreases.
(dissociation constant) increases then the value of hydrogen ion concentration also increases and the value of pH decreases.
That means, higher value of
 has lower pH and lower value of
has lower pH and lower value of
Hence, the solution that has highest hydronium ion concentration is, (B) HCN,