Answer:
efficiency of the engine is 0.4167
Step-by-step explanation:
given data
T high = 1200 K
T low = 700 K
solution
we get here efficiency that is express as
efficiency =
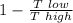 .........................1
.........................1
put here value of both temperature we get efficiency
efficiency = 1 -
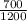
efficiency = 1 - 0.58333
efficiency = 0.4167
so efficiency of the engine is 0.4167