Answer:
The value of
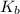 of the an ethylamine is
of the an ethylamine is
 .
.
Step-by-step explanation:
The pH of the solution = 12.067
The pOH of the solution = 14 - pH =14-12.607 =1.933
![pOH=-\log[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/aptpm2b2equoweomw80psbpn50765hcb2n.png)
![1.933=-\log[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/wlshvd5n3xeudejpmo7tsto26d1tmubwjq.png)
![[OH^-]=0.0117 M](https://img.qammunity.org/2021/formulas/chemistry/college/tbjcb3l8grbmyiypvuockv8avgvnhfiux6.png)
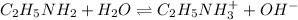
Initially
0.342 M 0 0
At equilibrium
(0.342-x) x x
The value of x =
![[OH^-]=0.0117 M](https://img.qammunity.org/2021/formulas/chemistry/college/tbjcb3l8grbmyiypvuockv8avgvnhfiux6.png)
The expression of
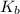 is given as:
is given as:
![K_b=([C_2H_5NH_3^(+)][OH^-])/([C_2H_5NH_2])](https://img.qammunity.org/2021/formulas/chemistry/college/66rvpohqhhg0trt79egurxgjjuuqah40ap.png)
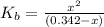
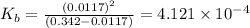
The value of
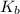 of the an ethylamine is
of the an ethylamine is
 .
.