Answer:
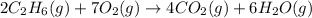
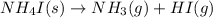
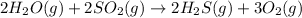
Step-by-step explanation:
Entropy is the measure of randomness or disorder of a system.
A system has positive value of entropy if the disorder increases and a system has negative value of entropy if the disorder decreases.
1.
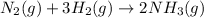
As 4 moles of gaseous reactants are changing to 2 moles of gaseous products, the randomness is decreasing and the entropy is negative
2.
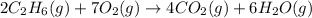
As 9 moles of gaseous reactants are changing to 10 moles of gaseous products, the randomness is increasing and the entropy is positive.
3.
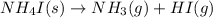
As 1 mole of solid reactants is changing to 2 moles of gaseous products, the randomness is increasing and the entropy is positive.
4.
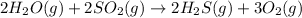
As 4 moles of gaseous reactants is changing to 5 moles of gaseous products, the randomness is increasing and the entropy is positive
5.
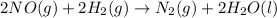
As 4 moles of gaseous reactants is changing to 1 moles of gaseous products, the randomness is decreasing and the entropy is negative.