- The chemical name for reactant side:
Al - Aluminium, Pb(NO3)2 - Lead(II) nitrate.
- The chemical name for product side:
Al(NO3)3 - Aluminium nitrate, Pb - lead.
Step-by-step explanation:
- This is an oxidation-reduction (redox) reaction:
3
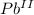 + 6 e- → 3
+ 6 e- → 3
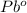 (reduction)
(reduction)
2
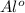 - 6 e- → 2
- 6 e- → 2
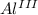 (oxidation)
(oxidation)
Pb(NO3)2 is an oxidizing agent, Al is a reducing agent.
Al uminium
Names: Aluminum, Aluminium powder, Al
Appearance: Silvery-white-to-grey powder, Silvery-white,
malleable, ductile, odorless metal.
Pb(NO3)2 – Lead(II) nitrate
Other names: Lead nitrate, Plumbous nitrate, Lead dinitrate
Appearance: White colorless crystals, White or colorless crystals
Al(NO3)3 – Aluminium nitrate
Other names: Nitric Aluminum salt, Aluminum nitrate,
Aluminium(III) nitrate
Appearance: White crystals, solid | hygroscopic
Pb - lead
Names: Lead, Lead metal, Plumbum
Appearance: Bluish-white or silvery-grey solid in various forms.
Turns tarnished on exposure to air. A heavy, ductile,
soft, gray solid.