Answer:
The molecular formula of the compound is
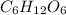 .
.
Step-by-step explanation:
Let the molecular formula of the compound is
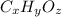 .in
.in
Molar mass of compound = 180 g/mol
Number of carbon atom = x
Number of hydrogen atom = y
Number of oxygen atom = z
Atomic mass of carbon = 12 g/mol
Atomic mass of hydrogen = 1 g/mol
Atomic mass of oxygen = 16 g/mol
Percentage of element in compound :
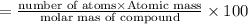
Carbon :
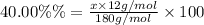
x = 46
Hydrogen :
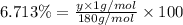
y = 12
Oxygen:
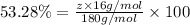
z = 5.99 ≈ 6
The molecular formula of the compound is
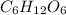 .
.