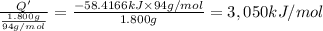Answer:
The balanced chemical equation:
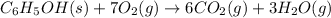
Heat of combustion per gram of phenol is 32.454 kJ/g
Heat of combustion per gram of phenol is 3,050 kJ/mol
Step-by-step explanation:
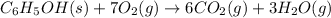
Heat capacity of calorimeter = C = 11.66 kJ/°C
Initial temperature of the calorimeter =
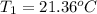
Final temperature of the calorimeter =
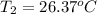
Heat absorbed by calorimeter = Q
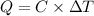
Heat released during reaction = Q'
Q' = -Q ( law of conservation of energy)
Energy released on combustion of 1.800 grams of phenol = Q' = -(58.4166 kJ)
Heat of combustion per gram of phenol:
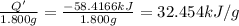
Molar mass of phenol = 94 g/mol
Heat of combustion per gram of phenol: