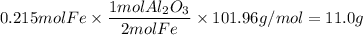Answer:
- 1. Iron(III) oxide: 17.2 g
Step-by-step explanation:
1. Chemical reaction (given)
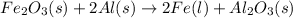
2. Mole ratios
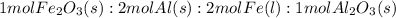
3. Convert 12.0g of iron to moles
- atomic mass of Fe: 55.845g/mol
- number of moles = mass in grams / atomic mass
- number of moles = 12.0g / 55.845g/mol = 0.215mol
4. Iron(III) oxide
- molar mass Fe₂O₃: 159.69 g/mol
- mole ratio: 1 mol Fe₂O₃ : 2 mol Fe
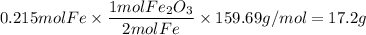
5. Aluminum
- atomic mass Al: 26.982g/mol
- mole ratio: 2 mol Al : 2 mol Fe
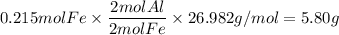
6. Aluminum oxide
- molar mass Al₂O₃: 101.96g/mol
- mole ratio: 2mol Fe: 1 mol Al₂O₃