Step-by-step explanation:
The reaction equation for this reaction is as follows.

The given data is as follows.
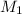 = ?,
= ?,
 = 20 ml
= 20 ml
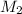 = 0.859 M,
= 0.859 M,
 = 10.1 ml
= 10.1 ml
Hence, we will calculate the original concentration of HCl as follows.
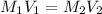
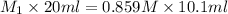
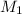 = 0.434 M
= 0.434 M
Thus, we can conclude that the original concentration of the HCl is 0.434 M.