Answer:
Liquid B
Step-by-step explanation:
The amount of heat energy that must be supplied to a substance in order to increase its temperature is given by:
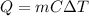
where
m is the mass of the substance
C is the specific heat capacity of the substance
 is the increase in temperature
is the increase in temperature
The equation can be rewritten as
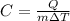
In this problem, we know that:
- Liquid A and B have same mass,
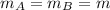
- The two liquids have same initial temperature, but afterwards liquid A is hotter than liquid B; this means that
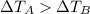
- Moreover, the two liquids are heated for the same time over identical burners: this means that the amount of heat supplied is the same for the two liquids,
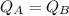
By looking at the equation, we see that since Q and m are the same for the two liquids, then their specific heat is just inversely proportional to the change in temperature: and since the change in temperature is larger for liquid A, this means that the specific heat is larger for liquid B.