Methane - 349 kJ
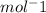 , Ethene - 681 kJ
, Ethene - 681 kJ
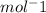 , and Ethyne - 815 kJ
, and Ethyne - 815 kJ
Step-by-step explanation:
The difference in the energy involved in carbon single bond, double bond, and triple bond are as mentioned below-
- Methane - 349 kJ
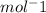
- Ethene - 681 kJ
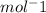
- Ethyne - 815 kJ
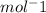
- These differences in energy depend on the number of bonds formed, bond lengths, sizes of atoms that are involved in the bond formation, affinities of the electron, electronegativity differences.
Hence, the shorter the bond length, the higher is the bond energy .
- The bonds in double and triple bonds that are formed in ethene and ethyne respectively are shorter than the bonds formed in single bonds formed in methane.
- Ethyne has the highest bond energy and methane has the lowest bond energy.