Answer:
1.274 moles
Step-by-step explanation:
The equation for the reaction can be represented as follows:
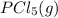 ⇄
⇄
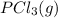 +
+
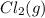
K = 0.060
K =
![([PCl_3][Cl_2])/([PCl_5])](https://img.qammunity.org/2021/formulas/chemistry/high-school/wuida3lp9sdoisjwaruv5zs9ubfwobk69f.png)
Concentration of
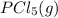 =
=
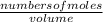
Concentration of
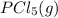 =
=
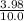
Concentration of
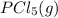 = 0.398 moles
= 0.398 moles
If we construct an ICE table for the above equation; we have:
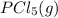 ⇄
⇄
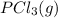 +
+
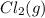
Initial 0.398 0 0
Change - x + x + x
Equilibrium (0.398 - x) x x
K =
![([PCl_3][Cl_2])/([PCl_5])](https://img.qammunity.org/2021/formulas/chemistry/high-school/wuida3lp9sdoisjwaruv5zs9ubfwobk69f.png)
K =
![([x][x])/([0.398-x])](https://img.qammunity.org/2021/formulas/chemistry/high-school/tq84xikx0bzd6fjcplzcbmbk4ykvhzn3j1.png)
K =
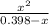
0.060 =
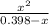
0.06(0.398-x) = x²
0.02388 - 0.060x = x²
x² + 0.060x - 0.02388 = 0 (quadratic equation)
a = 1; b= 0.06; c= -0.02388
Using quadratic formula;
=
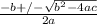
=
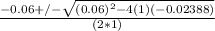
=
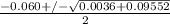
=
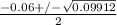
=
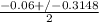
=
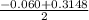 or
or
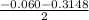
=
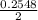 or
or
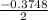
= 0.1274 or -0.1874
We go by the positive value which says:
[x] = 0.1274 M
number of moles = 0.1274 × 10.0
= 1.274 moles
∴ the number of moles of Cl₂ produced at equilibrium = 1.274 moles