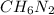Answer:

Step-by-step explanation:
1. First determine the empirical formula.
a) Base: 100 g of compound
mass atomic mass number of moles
g g/mol mol
C 26.06 12.011 26.06/12.011 = 2.17
H 13.13 1.008 13.13/1.008 = 13.03
N 60.81 14.007 60.81/14.007 = 4.34
b) Divide every number of moles by the smallest number: 2.17
mass number of moles proportion
C 2.17/2.17 1
H 13.03/2.17 6
N 4.34/2.17 2
c) Empirical formula
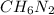
d) Mass of the empirical formula
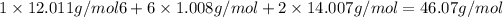
2. Molecular formula
Since the mass of one unit of the empirical formula is equal to the molar mass of the compound, the molecular formula is the same as the empirical formula: