Answer:
The molecular formula is most likely to be first option,
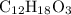 .
.
Explanation:
Both the molecular formula and the empirical formula of a molecule give the types of atoms in that molecule. However, the molecular formula of a molecule gives the exact number of atoms each molecule. On the other hand, the empirical formula gives only a simplified ratio.
For example, if the empirical formula of the molecule is
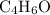 , then the molecular formula would be
, then the molecular formula would be
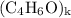 , or equivalently
, or equivalently
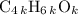 , where
, where
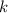 is a positive whole number (
is a positive whole number (
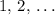 , etc.) The goal here is to find the value of
, etc.) The goal here is to find the value of
Look up the relative atomic mass data on a modern periodic table:
- C: 12.011.
- H: 1.008.
- O: 15.999.
The formula mass of
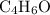 would be
would be
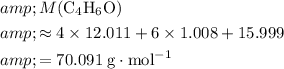 .
.
The formula mass of
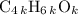 would be
would be
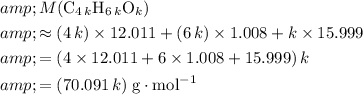 .
.
On the other hand, since the molecule should have a molecular mass of
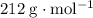 ,
,
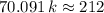 .
.
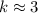 . (Round to the nearest whole number.)
. (Round to the nearest whole number.)
Hence, the molecular formula would be
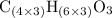 , which simplifies to
, which simplifies to
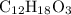 .
.