Answer:
its mass
its temperature
Step-by-step explanation:
The heat capacity of a body is the amount of heat needed to raise that temperature of a body by 1k or 1°C.
It is expresses as:
H = m c Ф
where m is the mass of the body
c is the specific heat of the body
Ф is the temperature change of the body
Specific heat capacity is that heat content needed to raise the temperature of a unit mass of a substance by 1k or 1°C
Heat capacity is also known as heat content;
To solve the problem:
since H = m c Ф
we make the unknown c the subject of the expression;
c =
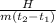 = H / mФ
= H / mФ
So to obtain the specific heat capacity, divided the heat capacity by mass and its temperature change.