Answer:
There are three laws that explain the behaviour of ideal gases:
1) Boyle's law:
"For a constant mass of ideal gas kept at constant temperature, the pressure of the gas is inversely proportional to the gas volume"
Mathematically:
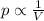
where
p is the pressure of the gas
V is its volume
An example of application of Boyle's law is in the gas inside a syringe: if we block the nozzle of the syringe, and we push on the other side, we compress the volume of the gas inside; as a result, we notice that it becomes more and more difficult to push the other side of the syringe: this is because the pressure of the gas inside is increasing, due to Boyle's law.
2) Charle's Law:
"For a constant mass of an ideal gas kept at constant pressure, the volume of the gas is proportional to its absolute temperature"
Mathematically:
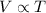
where
V is the volume of the gas
T is its absolute temperature (in Kelvin)
An easy example of application of Charle's law is a helium balloon. If we fill a balloon with helium gas, then we bring the ballon to another place with lower temperature, we notice that the balloon shrinks: this is because the temperature of the gas has decreased, and therefore the volume of the balloon decreases as well. Vice-versa, if we bring the balloon to a warmer place, it expands: the temperature has increased, and therefore the volume increases as well.
3) Gay-Lussac's law:
"For a constant mass of an ideal gas kept at constant volume, the pressure of the gas is proportional to its absolute temperature"
Mathematically:
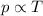
where
p is the pressure of the gas
T is the absolute temperature of the gas (in Kelvin)
An example of Gay-Lussac's law in daily life is a canister containing gas. The canister has a fixed volume, so does the gas inside. If the canister is placed above a flame, heat is transferred to the gas inside the canister: as result, the temperature of the gas increases. According to Gay-Lussac's law, the pressure increases as well: therefore, at some point, the pressure of the gas will be large enough to break the canister, which will then explode.