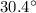The final temperature of the bath water is
Solution:
From given,
Mass of the hot water = m = 3 kg
Mass of cold water = M = 27 kg
Initial temperature of the hot water =
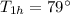
Initial temperature of the cold water =
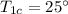
The heat lost by the hot water will be equal to the heat gain by the cold water
Therefore,
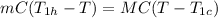

Thus the final temperature of the bath water is