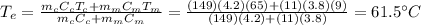Answer:
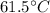
Step-by-step explanation:
When the cold milk is added into the hot coffee, heat is transferred from the coffee (higher temperature) to the milk (lower temperature), until the two substances are at the same temperature (thermal equilibrium).
Therefore, the heat given off by the coffee is equal to the heat absorbed by the milk; so, we can write:
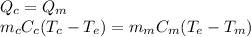
where:
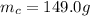 is the mass of coffee
is the mass of coffee
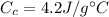 is the specific heat capacity of coffee
is the specific heat capacity of coffee
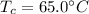 is the initial temperature of the coffee
is the initial temperature of the coffee
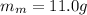 is the mass of milk
is the mass of milk
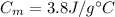 is the specific heat capacity of milk
is the specific heat capacity of milk
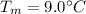 is the initial temperature of milk
is the initial temperature of milk
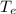 is the temperature at equilibrium
is the temperature at equilibrium
Solving for
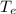 , we find the final temperature of the coffee at equilibrium:
, we find the final temperature of the coffee at equilibrium: