Answer:
The final temperature will be 22.52°C.
Step-by-step explanation:
Let the final temperature be T°C.
Heat gained by water = 120 × 4.186 × (T - 20)
= 502.32 (T-20)
Heat lost by iron nails = 100 × 0.460 × (50 -T)
= 46 (50-T)
As we know,
Heat lost = Heat gained
or, 46 (50-T) = 502.32 (T-20)
or, 2300 - 46T = 502.32T - 10046.4
or, (2300 + 10046.4) = (502.32 + 46) T
or, T =
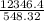
∴ T = 22.52°C
Hence the final temperature of the system is 22.52°C.