Answer:
Electrons are kept in the orbit around the nucleus by the electromagnetic force.
Step-by-step explanation:
- Electron revolves around the nucleus with a negative charge and the nucleus possesses a positive charge. The electrostatic force of attraction must pull the electrons into the nucleus but it's not that simple.
- There is a quantum mechanical feature that prevents the electrons to fall into the nucleus. This is described by the uncertainty equation between the position and momentum of the electron.
- The equation is represented as:
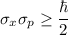
where,
ħ= reduced Planck constant
- This equation gives the limitation that an electron cannot fall into the nucleus.