Answer:
3.5 mol·L⁻¹
Step-by-step explanation:
1. Set up an ICE table.
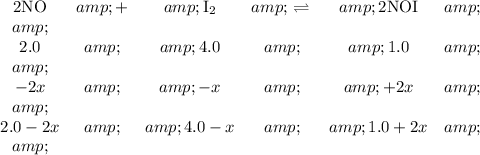
2. Solve for x
The equilibrium concentration of NO is 1.0 mol·L⁻¹, so
1.0 = 2.0 - 2x
2x + 1.0 = 2.0
2x = 1.0
x = 0.5
3. Calculate the equilibrium concentration of I₂
[I₂] = 4.0 - x = 4.0 - 0.5 = 3.5 mol·L⁻¹