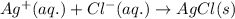Answer:
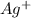 is present in solution
is present in solution
Step-by-step explanation:
In presence of HCl, metal ions present in solution will be precipitated as metal chloride provided these salts are either insoluble or sparingly soluble in water.
According to solubility rule, all chlorides are soluble except AgCl,
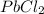 and
and
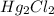
In all probability, addition of HCl in solution will result formation of AgCl,
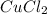 and
and
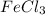 . But only AgCl is insoluble. Hence it will get precipitated out of solution.
. But only AgCl is insoluble. Hence it will get precipitated out of solution.
So,
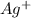 is present in solution
is present in solution
Balanced reaction: