Step-by-step explanation:
a) The standard molar enthalpy of denaturation of protein = ΔH = 512 kJ/mol
The standard molar entropy of denaturation of protein = ΔS = 1.60 kJ/mol K
The Gibbs free energy is given by ;
ΔG = ΔH - TΔS
- If the value ,ΔG < 0, then reaction is spontaneous.
- If the value ,ΔG > 0, then reaction is spontaneous.
Here in the question value ΔH and ΔS are positive and independent of temperature.
This means that value of Gibbs free energy depends only upon temperature that is at lower denaturation of protein will be non spontaneous and at high temperatures denaturation of protein will spontaneous.
b) The standard molar free energy of denaturation of protein at 25°C.
T = 25°C = 25+273 K = 298 K
ΔG = ΔH - TΔS
= 512 kJ/mol - 298 K × (1.60 kJ/mol K) = 35.2 kJ/mol
c) If the value ,ΔG < 0, then reaction is spontaneous.
Putting , ΔG = 0
ΔG = ΔH - TΔS
0 =512 kJ/mol - T × (1.60 kJ/mol)
512 kJ/mol =T × (1.60 kJ/mol K)
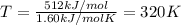
So, this means that above 320 Kelvins the denaturation of protein will be spontaneous.