The best interpretation of the lower value for O is that : The half-filled set of p orbitals in N makes it more difficult to remove an electron from N than from O
Step-by-step explanation:
The potential for ionized electrons is a measure of how quickly an atom is destroyed.
The capacity for ionization is increased around the table from left to right. The actual electronic N atom structure is 2
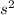 2
2
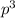 (half-filled orbital), Which allows it additional stability.
(half-filled orbital), Which allows it additional stability.
This increases the energy required to remove the first electron from N in comparison with O.