Answer:
(1) Magnesium carbonate :-
![Q_(MgCO_3)=[Mg^(2+)][CO_3^(2-)]](https://img.qammunity.org/2021/formulas/chemistry/college/4hwd90in7op1tgwswdtqwhwrwv7gi62no2.png)
(2) Calcium phosphate :-
![Q_(Ca_3(PO_4)_2)=[Ca^(2+)^3][PO_4^(3-)^2]](https://img.qammunity.org/2021/formulas/chemistry/college/ahu6qgx4gbxqqv8o4eowgnspk1be5c3s49.png)
(3) Silver sulfide :-
![Q_(Ag_2S)=[Ag^(+)^2][S^(2-)]](https://img.qammunity.org/2021/formulas/chemistry/college/wyo2549vmak6iscnona7k8u6asvpxb88es.png)
Step-by-step explanation:
(1) Magnesium carbonate will dissociate as:-
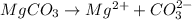
The expression for the ion-product is as follows:-
![Q_(MgCO_3)=[Mg^(2+)][CO_3^(2-)]](https://img.qammunity.org/2021/formulas/chemistry/college/4hwd90in7op1tgwswdtqwhwrwv7gi62no2.png)
(2) Calcium phosphate will dissociate as:-
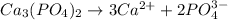
The expression for the ion-product is as follows:-
![Q_(Ca_3(PO_4)_2)=[Ca^(2+)^3][PO_4^(3-)^2]](https://img.qammunity.org/2021/formulas/chemistry/college/ahu6qgx4gbxqqv8o4eowgnspk1be5c3s49.png)
(3) Silver sulfide will dissociate as:-
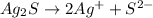
The expression for the ion-product is as follows:-
![Q_(Ag_2S)=[Ag^(+)^2][S^(2-)]](https://img.qammunity.org/2021/formulas/chemistry/college/wyo2549vmak6iscnona7k8u6asvpxb88es.png)