Answer:
For a: The mixture will need to produce more reactants to reach equilibrium.
For b: The mixture will need to produce more reactants to reach equilibrium.
For c: The mixture will need to produce more products to reach equilibrium.
Step-by-step explanation:
For the given chemical equation:
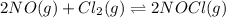
The expression of
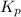 for above equation follows:
for above equation follows:
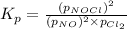 .....(1)
.....(1)
We are given:
Value of
 = 0.26
= 0.26
There are 3 conditions:
- When
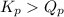 ; the reaction is product favored.
; the reaction is product favored. - When
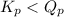 ; the reaction is reactant favored.
; the reaction is reactant favored. - When
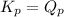 ; the reaction is in equilibrium.
; the reaction is in equilibrium.
For the given options:
We are given:
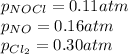
Putting values in expression 1, we get:
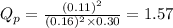
As,
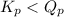 ; the reaction is reactant favored
; the reaction is reactant favored
Hence, the mixture will need to produce more reactants to reach equilibrium.
We are given:
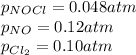
Putting values in expression 1, we get:
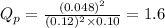
As,
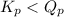 ; the reaction is reactant favored
; the reaction is reactant favored
Hence, the mixture will need to produce more reactants to reach equilibrium.
We are given:
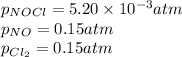
Putting values in expression 1, we get:
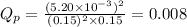
As,
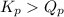 ; the reaction is product favored.
; the reaction is product favored.
Hence, the mixture will need to produce more products to reach equilibrium.