Answer:
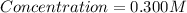
Step-by-step explanation:
This question tests for concentration after dilution of the solution.
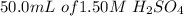 is added to 200ml of water.
is added to 200ml of water.
The formula for concentration correlation is given as
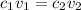 , where the latter relates to the final solution mix.
, where the latter relates to the final solution mix.
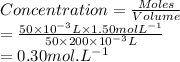
Therefore the concentration is 0.300M