The given question is incomplete. The complete question is as follows.
Solid vanadium crystallizes in a body-centered cubic structure and has a density of 6.00 g/cm3. Assuming the vanadium atomic radius is 132 pm, is the vanadium unit cell primitive cubic, body centered cubic, or face centered cubic.
Step-by-step explanation:
The given data is as follows.
Density = 6.00
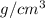
radius = 132 pm
Relation between edge length and volume is as follows.
a = ∛V
= ∛No. of atoms
=
![\sqrt[3]{(mass)/(density * N_(A))}](https://img.qammunity.org/2021/formulas/chemistry/college/v2bcoqzexjjssktv7zafg180no7pxpr8be.png)
=
![\sqrt[3]{(50.941 g/mol)/(6.0 g/cm^(3) * 6.022 * 10^(23))}](https://img.qammunity.org/2021/formulas/chemistry/college/g90eh44erzstl0ehhu3x6yu24y2vitfa08.png)
= 2.4 ∛No. of atoms
So, there will be three possibilities which are as follows.
- SC, 1 atom and r =
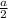
Here, a = 2.4, and r = 1.2
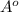 which is not right.
which is not right.
- For BCC, there are two atoms and r =
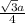
So, a =
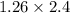
= 3.02 and, r = 1.31
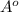 which is a good fit to the measured radius.
which is a good fit to the measured radius.
- For FCC, there are 4 atoms and r =
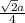
So, a =
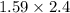
= 3.81 and r = 1.35
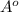 which will not fit to the measured radius as well as BCC.
which will not fit to the measured radius as well as BCC.