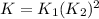The question is incomplete, complete question is :
The first two steps in the industrial synthesis of nitric acid produce nitrogen dioxide from ammonia:
Step 1 :
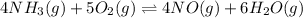
Step 2 :
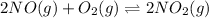
The net reaction is:
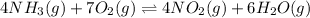
Write an equation that gives the overall equilibrium constant K in terms of the equilibrium constants
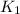 and
and
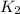 . If you need to include any physical constants, be sure you use their standard symbols
. If you need to include any physical constants, be sure you use their standard symbols
Answer:
Equation that gives the overall equilibrium constant K in terms of the equilibrium constants
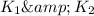 :
:
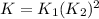
Step-by-step explanation:
Step 1 :
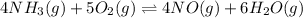
Expression of an equilibrium constant can be written as:
![K_1=([NO]^4[H_2O]^6)/([NH_3]^4[O_2]^5)](https://img.qammunity.org/2021/formulas/chemistry/college/ewh48k90osybfzdsw6peuhn7w2dp3ztmne.png)
Step 2 :
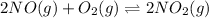
Expression of an equilibrium constant can be written as:
![K_2=([NO_2]^2)/([NO]^2[O_2])](https://img.qammunity.org/2021/formulas/chemistry/college/b6z63x7u9zmrdvy4rhkf2ydu60i1upcwgx.png)
The net reaction is:
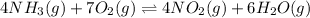
Expression of an equilibrium constant can be written as:
![K=([NO_2]^4[H_2O]^6)/([NH_3]^4[O_2]^7)](https://img.qammunity.org/2021/formulas/chemistry/college/arljswca8ou30bxo4o6n5q5uhanlga7frr.png)
Multiply and divide
![[NO]^4](https://img.qammunity.org/2021/formulas/chemistry/college/qxn3efo6q5qx9eq0h0dkemqdynbt6y9u0k.png) ;
;
![K=([NO_2]^4[H_2O]^6)/([NH_3]^4[O_2]^7)* ([NO]^4)/([NO]^4)](https://img.qammunity.org/2021/formulas/chemistry/college/pillab7cfezj2702lk04j9pt7zawiqdfeh.png)
![K=([NO]^4[H_2O]^6)/([NH_3]^4[O_2]^7)* ([NO_2]^4)/([NO]^4)](https://img.qammunity.org/2021/formulas/chemistry/college/rx8wt7m4hbgdk3tm9vhf5ckgbo53f9rvo3.png)
![K=K_1* ([NO_2]^4)/([O_2]^2[NO]^4)](https://img.qammunity.org/2021/formulas/chemistry/college/uu18mhcu5lk8h5r3bu5p21coc9ygb6y3yh.png)
![K=K_1* (([NO_2]^2)/([O_2]^1[NO]^2))^2](https://img.qammunity.org/2021/formulas/chemistry/college/q7r3nazgkyo3775rah9gt0bjm82b5gego5.png)
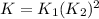
So , the equation that gives the overall equilibrium constant K in terms of the equilibrium constants
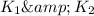 :
: