This is an incomplete question, here is a complete question.
The given chemical reaction is:
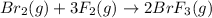
The equilibrium constant for the reaction below, at a given temperature is 45.6. If the equilibrium concentrations of F₂ and BrF₃ are 1.24 × 10⁻¹ M and 1.99 × 10⁻¹ M respectively, calculate the equilibrium concentration of Br₂.
Answer : The equilibrium concentration of Br₂ is, 0.0428 M
Explanation : Given,
Concentration of
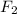 at equilibrium =
at equilibrium =
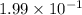
Concentration of
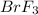 at equilibrium =
at equilibrium =
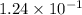
Equilibrium constant = 45.6
The given chemical reaction is:
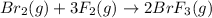
The expression for equilibrium constant is:
![K_c=([BrF_3]^2)/([Br_2][F_2]^3)](https://img.qammunity.org/2021/formulas/chemistry/college/vt9ul0wt31nh7pzwtxwcrebvquw7xvo5jf.png)
Now put all the given values in this expression, we get:
![45.6=((1.24* 10^(-1))^2)/([Br_2]* (1.99* 10^(-1))^3)](https://img.qammunity.org/2021/formulas/chemistry/college/twlqrmlp7mdjp4eqddj02lpsi3z0yohbpe.png)
![[Br_2]=0.0428M](https://img.qammunity.org/2021/formulas/chemistry/college/otv0vz03fc9vowpntxqjvrq4orsav8ljos.png)
Thus, the equilibrium concentration of Br₂ is, 0.0428 M