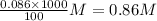Answer:
molarity of ethanol in beer =
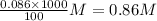
Step-by-step explanation:
5% ethanol in beer by volume means there are 5 mL of ethanol in 100 mL of beer.
Density is the ratio of mass to volume and
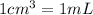
Mass of 5 mL of ethanol =
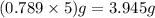
Number of moles is the ratio of mass to molecular weight
So, 5 mL of ethanol =
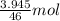 of ethanol = 0.086 mol of ethanol
of ethanol = 0.086 mol of ethanol
Molarity represents number of moles of solute present in 1000 mL of solution.
Here solute is ethanol
So, molarity of ethanol in beer =