Answer:
The mass remains after 27.5 days is 8.043 mg.
Step-by-step explanation:
Given that:
Half life = 14.3 days
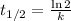
Where, k is rate constant
So,
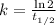
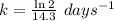
The rate constant, k = 0.04847 days⁻¹
Time = 27.5 days
Using integrated rate law for first order kinetics as:
![[A_t]=[A_0]e^(-kt)](https://img.qammunity.org/2021/formulas/chemistry/high-school/p2vpxs9cve2w798wzy926mzqzvfv5si8xr.png)
Where,
![[A_t]](https://img.qammunity.org/2021/formulas/chemistry/high-school/c6se0yk0a5jz0ud2m1a9jh5tv0rk9jx59i.png) is the concentration at time t
is the concentration at time t
![[A_0]](https://img.qammunity.org/2021/formulas/chemistry/high-school/i49y9xugeve1tuhjmf05tpufcmfey5f0yu.png) is the initial concentration = 30.5 mg
is the initial concentration = 30.5 mg
So,
![[A_t]=30.5* e^(-0.04847* 27.5)\ mg=8.043\ mg](https://img.qammunity.org/2021/formulas/chemistry/college/jatrgyqymltm7i63mp0fm78g8tmt8va1iv.png)
The mass remains after 27.5 days is 8.043 mg.