Answer:
T = 11.4°C
Step-by-step explanation:
Given that:
weight of solute w (glucose) = 12 g
weight of solvent W (acetic acid) = 50 g
Molar depression constant of solvent (Kf)= 3.90°C/m
Temperature of solvent freezing point T₀ = 16.6°C
Temperature of solution freezing point Tₓ = (T₀ -T) = ???
Molar mass of solute (glucose) = 180.2 g/mol
Using the expression;
(T₀ -T) =
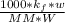
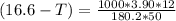
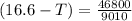
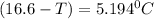
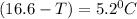
-T = -16.6 °C + 5.2 °C
- T = -11.4°C
T = 11.4°C
∴ The freezing point of the solution is said to be = 11.4°C